Rare diseases (RDs) are generally defined as affecting one in every 20,000, with 5,000–8,000 distinct conditions affecting millions globally.1 Diagnostic complexity and lack of awareness often lead to delayed diagnosis and treatment, averaging 5.6–7.6 years with poor outcomes.2 Centralizing expertise through specialized centres can improve diagnosis and outcomes, advance research and support patient advocacy. Global initiatives, such as those by the World Health Organization (WHO) Rare Disease Initiative (RDI) and the International Rare Diseases Research Consortium (IRDiRC), aim to empower primary healthcare providers to improve patient outcomes.3 Several initiatives worldwide coordinate information on RDs and promote multidisciplinary collaboration; yet, attention to clinic initiation is limited.4 Connecting with a RD clinic can be a transformative and hopeful step in the patient journey. While setting up such a clinic may seem complex and challenging, navigating the specialized care challenges of a RD can be deeply rewarding for the treating team and patients alike. This narrative article highlights considerations in the initiation and growth of RD clinics, with myositis diseases as an example. Several key concepts permeate throughout this article such as incremental growth based on available resources, being informed by the patient community, fortifying local and global collaborations with services and colleagues, inter-collegial learning and early career mentorship.
Unmet needs in rare diseases
Individuals with prevalent diseases often benefit from well-defined screening and management guidelines supported by substantial evidence. The journey of people living with RDs (PLWRDs) however carries prolonged diagnostic delays marked by repeated misdiagnoses. Upon diagnosis, a paucity of evidence-based therapeutic options and sufficiently experienced specialists hinder the initiation of appropriate treatment.5
Dedicated RD clinics assess and prioritize critical disease-specific needs through access to multidisciplinary specialists, emerging targeted therapies and disease-specific patient education and counselling.5 Due to the geographically dispersed nature of RD populations, recruiting patients for any type of research is a major challenge. Thus, RD clinics often facilitate patient enrolment in multi-centre clinical trials for the expansion of therapeutic options and other collaborative research to elucidate disease natural history as well as patient disease experience and insight.
RD management is frequently challenged by heavy reliance upon consensus guidelines weighted heavily on ‘expert’ experience rather than high-level evidence. However, this challenge is often compensated by the specialist’s dedicated experience and careful thought given to these disease manifestations, symptoms and complications. Importantly, RD clinics potentially provide PLWRDs and their families with the reassurance of experienced care and connection with others living with the same condition, which can often lessen the burden of isolation and lend mutual support to navigate living with an RD through shared experiences.
Incremental development of rare disease clinics
Most RD clinics have evolved organically from the seeds of clinician interest that naturally attract patient cohorts with operational throughputs subsequently taking root over time. Immediate engagement with the RD population and cultivating resources and teams over time, rather than postponing the RD initiative for elaborate strategic planning, allows for the cultivation of clinical expertise, a chance to grow patient cohorts and provision of much-needed RD patient care. RD services typically develop over time, guided by the holistic needs of patients and available resources.
The evolution of specialized clinics from within generalist clinical settings represents the developmental pathway in most healthcare services. In the case of myositis, RD clinics initially arise from within a general speciality clinic, e.g. neurology, rheumatology, dermatology, among others. In the authors’ experience, they then begin to take root through organic processes and planning that are responsive to available resources and patient needs. As an example, a clinician may begin seeing a cluster of patients with RDs within their general clinic and take a strong interest in the patient journey or other related aspects and gradually develop specialized expertise through repeated encounters and increasingly higher number of patients, eventually leading to dedicated clinic sessions.
This evolutionary model differs fundamentally from top–down implementations in its feasibility and immediacy as it arises from frontline clinical encounters and responsiveness to patient needs. Such clinics often establish robust patient-centred approaches before receiving institutional recognition, thus demonstrating the value of allowing clinical innovation to emerge from practice and multi-disciplinary working rather than solely through strategic planning initiatives.6 The evolution typically progresses through several avenues of visibility: patients gravitating towards the clinician with specialized interest, the clinician’s growing specialist knowledge and skills bank, increasing referrals from colleagues and seeding of local multidisciplinary relationships, which ideally, but not always, lead to formal healthcare system recognition and dedicated resource allocations. For instance, the Multidisciplinary Team Consultation Program at Peking Union Medical College Hospital in China exemplifies a patient-centric model that enhances diagnostic and therapeutic strategies through coordinated care.7
This strategy, used by the Comprehensive Rare Disease Care model implemented at a South Indian RD clinic, demonstrates how meticulous planning and interdisciplinary collaboration can establish functional RD units even in resource-limited settings.8 However, as many RDs do not generate large revenue for an institution, many RD clinics, despite great visibility and public-facing success, may find attaining sufficient institutional support challenging.
In the authors’ experience, a RD clinic often gains momentum through phased growth in relation to its unique resources, feasibility and patients. The goals are to incrementally work towards clinical, research and financial sustainability and growth. Thus, outlining key milestones within specified, but flexible, timelines may help prioritize goals and establish smooth operations as new clinics grow (Figure 1).
Figure 1: A sample timeline for myositis clinic development
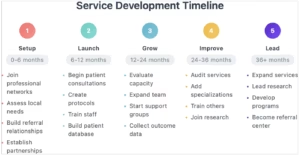
Registering the clinic in its early stages with relevant patient organizations and research networks and disease-specific groups such as, in the case of myositis, the Myositis International Health and Research Collaborative Alliance (MIHRA), the International Myositis Assessment and Clinical Studies Group (IMACS), The Myositis Association, the Cure Juvenile Myositis Foundation, World Muscle Society and the European Neuromuscular Centre or with broader RD alliances such as Rare Diseases Europe, National Organization of Rare Disorders (NORD), Global Genes and also RD-Connect can significantly increase visibility, facilitate access to expert networks and enhance patient referral pathways. Such engagement also strengthens outreach, advocacy and opportunities for collaborative research.9–18
Setting up basic clinic operations might incorporate intake protocols, disease-relevant patient-reported and clinical exam assessments and, possibly, available genetic and biomarker-based evaluations. Early integration into the electronic health records (EHR) for data tracking is ideal but not necessary. Many successful RD clinics began their patient databases with spreadsheets, and many years later, they transferred data to more sophisticated records.
Over time, the clinic is expected to expand its scope to include standardized treatment protocols based on the clinic’s experience and literature review. The integration of telemedicine services, particularly after the COVID-19 pandemic, will enhance access for remote patients. Educational initiatives targeting both healthcare providers and patients will further strengthen services. The holistic development of early career consultants through mentorship programs and regular continuing medical education for clinic staff can strengthen team dynamics and service quality – and many of these resources are offered cost-free.9,19 Cyclical quality assessment, funding acquisition and policy advocacy remain essential for long-term sustainability. Policy advocacy plays a critical role in influencing healthcare policy, improving patient access to treatments and securing institutional and government support for RD services. As the clinic evolves, collaboration with others in the field and possibly participating in clinical trials are likely to become the natural next steps.
Local patient support groups (PSGs) enhance care by connecting patients with experienced peers who share practical self-management strategies. Setting up PSGs either in-person, online or hybrid and either locally or regionally supports patients and, most importantly, provides clinicians with intensified learning experiences about the RD in question that help shape the clinician into a patient-centred specialist – a priority for patients. Patient organizations often provide information booklets and signpost newly diagnosed patients to these groups that help maintain an active growth community.20,21 The feedback loop between PSGs and the RD clinic can also assist in identifying gaps in care delivery while providing vital emotional and practical support during the patient journey.
Paths paved in rare diseases: Illustrative examples of initiatives for myositis
Even established RD clinics continue to learn from each other and provide fresh insight on operational models for new, growing and well-established RD clinics. The ‘Casa dos Raros’ in Brazil is an example of an all-inclusive RD clinic that encompasses several RDs and operates as a non-profit organization model that demonstrates a significant reduction in the time to diagnosis.22 In addition to comprehensive multidisciplinary clinical and laboratory evaluation of patients, it has integrated on-site genomic testing.
Similarly, the West China Hospital of Sichuan University integrates 5G-based remote consultations to improve care for PLWRDs living in rural areas, offering a framework for myositis clinics.23 Leal et al. among many other publications consider key concepts in setting up a multidisciplinary clinic, such as funding and integrating services, which could inform the development of RD services.24–27 The multidisciplinary myositis clinic and systemic autoinflammatory diseases clinic at a tertiary referral centre in Portugal exemplifies a collaborative approach between different specialities to ensure accurate diagnosis, standardization of care and follow-up for myositis and systemic autoinflammatory diseases, while also advancing research.28,29
The myositis unit at Karolinska Institute emerged from modest beginnings and has expanded into one of the largest myositis research centres globally. The unit maintains a comprehensive MYONET registry with meticulous documentation of each patient encounter embedded in clinical workflows, prioritizing clinical practicality and integrating multidisciplinary data collection, including physiotherapy assessments. 30–32 The institute pioneered the sutureless muscle biopsy (SMBx) technique by adapting otolaryngological instruments, creating a safer, less-painful procedure that enables sequential biopsies for monitoring treatment response and disease progression.33,34
Another example of ingenuity is the MyoCite cohort in Northern India, which initiated a comprehensive biorepository of several hundred adults and children with myositis which started from minimal seed funding for autoantibody testing.35 Their structured case report form (CRF) facilitated systematic data collection of relevant disease elements, underscoring the substantial returns possible from being responsive to population needs, available resources and feasible clinic planning and setup.36 The initiative’s success centred on a biobank seeded by a small extramural grant that embedded CRF with dense data capture, allowing clinical scientists to answer several research questions while establishing a cohort for future follow-up studies.35
The West Midlands myositis service in the UK evolved from an initial pool of patients from various colleagues across two to three satellite centres into a comprehensive care centre. The service’s growth was catalysed by pathways such as sutureless muscle biopsy and a vision to coordinate medical encounters in as few visits as possible. It now integrates musculoskeletal ultrasound, neurology and neurophysiology review, electromyography studies, digital pathways with patient–reported outcome measures (PROMs) integration and multidisciplinary team (MDT) assessments including physiotherapy and occupational therapy.37 Their efforts are a model for how systematic service developments over time can evolve into integrated care delivery for a holistic approach to health and well-being.
There are many ways an RD clinic can be started as long as the dedicated interest to care for these patients is present. A further example of this point is RD clinics that arise from highly specialized clinics in a different condition. In myositis, a number of myositis clinics grew from speciality clinics in muscular dystrophy (MD). In particular, inclusion body myositis was present in MD clinics.38 This emerging care need led to the development of dedicated speciality clinics and sparked new research interests.
Individually, RDs are rare; thus, national and international collaborations are critical for advancing research as well as patient care. The European Scleroderma Trials and Research (EUSTAR) group, World Muscle Society, IMACS and MIHRA among many others exemplify communities that proactively support global site growth and multicentre collaborations in the study and also understanding of rare rheumatic diseases such as systemic sclerosis and myositis.9,39,40 Similarly, the Multicenter Networks for Ideal Outcomes of Pediatric Rare Endocrine and Metabolic Disease was initiated, with 30 centres studying rare endocrine diseases.41 The Rare Diseases Clinical Research Network (RDCRN) consortia have studied various metabolic and genetic RDs in paediatric population.42 Many professional societies maintain networks with RD partners such as the European Respiratory Society (ERS) and the American Thoracic Society (ATS).43,44 Collaborations between clinicians and scientists are also essential to understanding the mechanistic role of implicated genes in the pathogenesis of the RD. The Canadian Rare Diseases Models and Mechanisms (RDMM) Network and the National Institute of Health Undiagnosed Disease Program were established to create a collaboration between clinicians and scientists. Both these programmes have proven their efficacy in aiding the discovery of new potential therapies for RDs.45,46 Various national and international organizations and initiatives such as NORD, Global Genes, National Center for Advancing Translational Sciences–RDCRN (NCATS–RDCRN), WHO and International Rare Diseases Research Consortium are involved in various aspects of RDs such as research, national and international coordination and patient support and advocacy.16,17,47–49 Similarly, study groups such as MYONET, OMERACT Myositis Working Group, PReS JDM working group, EULAR myositis study group, MARC (Myositis Audit and Collaborative Network) and COVAD (Collating the Voice of autoimmune diseases) are focussed on researching patients with inflamatory myopathies.50–55
Multidisciplinary team
RD cases benefit from multiple minds reviewing and contributing insight into diagnostic assessment and treatment plans. Cultivating a well-organized MDT ensures comprehensive diagnostic approaches and disease management, reducing important concepts from being overlooked on a case-by-case basis.28 Coordinating multiple services in as few visits as possible along with effective communication of results and management plans among the MDT enables timely diagnosis and treatment. This approach reduces the burden on patients and caregivers while also being cost-effective.27,56–58 Several studies for diseases like Huntington’s disease and complex genetic syndromes emphasize the importance of a MDT in the management of patients with RDs.58–60 A systematic review of randomized controlled trials assessing patient satisfaction in relation to team-based care versus usual care reported team–based care as better in improving patient satisfaction.61
Specialists
Clearly defined roles, especially in assigning MDT member case accountability ensure coordination and follow–through of tasks and communication. MDT members responsible for coordinating cases are expected to be or become fluent in all types of diagnostic testing and services related to the RD which potentially includes imaging, patient-reported, clinician-performed, pathology and laboratory assessments.28 In a myositis clinic, an MDT might include various key specialists as shown in Figure 2. The team structure allows for subspecialization in specific myositis subtypes, enabling targeted therapeutic approaches and improved clinical trial participation while ensuring comprehensive support across all aspects of patient care.62 Clinics may allocate dedicated time slots or consultation rooms for members of the MDT, using a shared workspace and a coordinated consultation schedule to promote seamless collaboration. Locating related specialities such as neurology, rheumatology and physiotherapy in adjacent rooms can further enhance cross-consultation and integrated patient care. In addition to defining the core disciplines of the MDT, regular multidisciplinary case review meetings (e.g. monthly or biweekly) should be scheduled to review complex cases. These meetings provide a platform to review diagnostic uncertainties, align treatment plans and incorporate the perspectives of all members of the team. Structured case discussions, shared documentation and role clarity among team members enhance both clinical outcomes and continuity of care.
Figure 2: Members of the multidisciplinary team
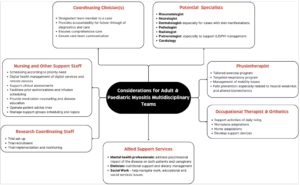
ILD = interstitial lung disease; PH = pulmonary hypertension
Diagnostic and laboratory partnerships
RD clinics require building collaborative relationships with laboratory, imaging and other diagnostics services to help develop efficient operational throughputs and effective communication channels with diagnostic teams that significantly enhances patient care quality and efficiency.48 Even without an ‘official centre’, such relations bring visibility to a clinic’s efforts and a better understanding of the needs of the patient community, which help to increase scheduling efficiency as well as quality patient interactions, procedural implementation and results reporting.
Laboratory assessments like autoantibody testing, using antinuclear antibody (ANA) as an example, vary significantly between centres in terms of dilutions, standardization methods or cut-off values for line blot assays, and these change without clinician awareness or do not include an opportunity for clinicians to weigh in on unusual patterns.63–65 Autoantibody testing in myositis also demonstrates variability in method applications, yielding false results.66–68 Investing in partnerships with diagnostics services increases the likelihood for standardized handling of samples as well as efficient expansion of rarer or newer antibodies such as TIF1-γ and MDA5, and it also increases their willingness to coordinate specialized testing unavailable locally or nationally. Further, in developing regions, where such automated laboratory alerts might not be available, establishing personal connections with laboratory staff ensures prompt communication of critical values to the appropriate clinical team.69,70 These relationships often lead to enhanced referral patterns and cohort expansion as service staff become more aware of the clinic’s specialization.
Imaging services are also enhanced by the above collaborative approaches. Additionally, cultivating the radiology team as central MDT members serves to address unmet educational needs to improve diagnostic accuracy through peer and trainee mentorship of non-radiology MDT members. As an example, the interpretation of muscle inflammation versus damage on magnetic resonance imaging demands specific expertise, involving training and structured radiology-led MDT discussions, that would enhance the diagnosis and management of myositis.71,72
Ultrasound (US) can be useful for diagnosing and subtyping myositis in settings where histopathology is not readily accessible and even for conducting procedures or identifying suitable biopsy sites.73–75 US’s non-invasive nature, increasing ease of availability and good ability to discriminate between myogenic and neurogenic and also some specific myopathies such as inclusion body myositis from close mimics can support patient follow up, especially in low–income settings.76–80 One major drawback is its subjective nature and need for training. Establishing coordinated partnerships between clinicians and imaging specialists early helps to develop standardized protocols and reporting templates to ensure consistent interpretation across different operators.
MDTs benefit from regular interactions and collaboration with pathology services. The inclusion of clinico-pathological discussions with supportive clinical context enhances diagnostic accuracy and assistance with the increasing identification of digenic diseases.81 Regional clinico-pathology MDTs are opportunities for valuable learning and diagnostic acumen of challenging cases and identification of myositis mimics.82
Participating in longitudinal data collection
A RD clinic serves as a centralized hub for patient care as well as collaborative research. The gradual accumulation of a well-characterized patient cohort enhances research opportunities, facilitating novel insights and the development of targeted treatments. To streamline research activities, recruiting dedicated research assistants and data entry personnel is a goal to increase the efficiency of data management and study coordination. A clinical CRF is pivotal for efficiency and standardized patient data collection for quality outcomes in RD clinical research and contributes to valuable insights into the natural history of and potential therapies in RDs like myositis.31,36,83–86 Longitudinal registry data combined with biobanking – the collection, storage and management of biological samples, such as blood, muscle tissue and DNA from patients, can contribute to remarkable clinical insights.
Biobanking, especially combined with clinical data, provides a gateway to understanding disease mechanisms and new biomarker discovery, thus aiding development of targeted and personalized therapies.31,86–88 General guidelines for bio-banking are provided by several bodies such as the International Society for Biological and Environmental Repositories (ISBER) and the International Standards Organization (ISO).89,90 Biobanks for rare rheumatic diseases have been set up by several international bodies.39,86 Similarly, a neuromuscular diseases biobank called the MRC Centre Biobank for Neuromuscular Diseases has been set up in London and Newcastle, and several biobanks have been set up for myositis specifically.35,86,91
Education
A RD clinic serves as a vital contribution to education for medical students, postgraduate trainees and postdoctoral fellows regardless of the fields they ultimately enter. Exposure to disease models and approaches to complex care under expert mentorship enables trainees to have unique experiences that benefit their understanding of common diseases. Further, cultivating early career interest in RDs ensures a stronger future for RD clinicians and researchers.
Mentorship is integral to research and training in a myositis clinic, fostering expertise in clinical care, diagnostics and translational research. Structured programmes such as IMACS and the MIHRA Career Enhancement Core support students, early-career clinicians and researchers through mentorship and skill development opportunities.9,10 Additionally, funding from the Rare Diseases Clinical Research Network (RDCRN), supported by the National Institutes of Health (NIH), ensures that trainee opportunities and education are prioritized in RDs, thus creating a commitment to cultivating the next generation of RD specialists.47 A structured approach to research and training creates a dynamic learning environment, bridging the gap between scientific discovery and clinical application while advancing patient care and professional development.
Feedback and quality improvement
Regular service evaluation through structured audits and governance reviews ensures continuous improvement in care delivery.92 Standard operating procedures (SOPs) should be periodically revised to incorporate both patient feedback and peer recommendations. For instance, the muscle biopsy pathway can be refined by systematically collecting patient experience surveys, tracking complications and analyzing workflow efficiency. This iterative approach to service development, combining clinical outcomes data with patient-reported experiences, helps identify bottlenecks and opportunities for enhancement. Regular MDT meetings can review these metrics and implement necessary adjustments, ensuring services remain responsive to patient needs while maintaining high clinical standards.
Provision of transitional care
As per the Lancet Global Health, almost 70% of RDs are present during childhood, and most of these diseases persist throughout life, requiring prolonged healthcare access.1 Adolescence is a crucial phase in the development of an individual, having an impact on the various domains of an individual’s life. Thus, in children with chronic diseases, this phase demands extra attention and care requiring a smooth transition from child-centred to adult-oriented systems.93
Studies across various diseases demonstrate that nearly half of paediatric patients are lost during transition, predisposing them to an increased risk of unfavourable outcomes.94–96 There is an unmet need for transitional care in paediatric patients with RDs, as outlined by the European Reference Network on Connective Tissue and Musculoskeletal Diseases (ERN ReCONNET) Transition of Care Task Force.97 In juvenile myositis, this is a critical concept as the disease burden and disability related to physical and psychological impact is high.98
Role of digital health technologies
Service expansion in myositis care requires a multifaceted approach combining digital innovation with physical outreach. Telemedicine and remote monitoring systems enable regular follow-up care and symptom tracking while reducing the burden of frequent hospital visits, which is particularly beneficial for patients with mobility challenges or those in remote areas.99 Kiefer et al. demonstrated that remote motor assessment is reliable, acceptable and feasible in children with ultrarare leukodystrophy, Canavan disease.100 Thus, in addition to providing patient care, telemedicine can also aid in carrying out decentralized trials, which would be of great benefit to RD research. Telemedicine can also improve quality communication between the patient and healthcare provider as patients feel more at ease in their familiar surroundings and can also acquaint the healthcare providers with the patient’s environment.101 This digital framework can be complemented by strategic outreach clinics that bring specialized care to underserved regions through mobile or satellite facilities. Local healthcare provider partnerships enhance these efforts by facilitating early detection and maintaining continuity of care.
In addition to aiding in patient care and monitoring, digital health technologies (DHTs) like telemedicine, electronic patient-reported outcomes, self-sampling devices, EHR and large language models (LLMs) reduce the hours and efforts invested in patient outreach, data collection and documentation. With limitations in the availability of the workforce, DHTs can aid in the judicial allocation of limited resources.102 Initiatives such as digital drug counselling, nurse-led digital cardiovascular assessments and pre-appointment patient-reported symptom surveys have been successfully implemented at The Royal Wolverhampton NHS Trust hospitals in England’s West Midlands, serving a geographically dense population with diverse ethnicities and a wide spectrum of disease types. Other initiatives include periodic PROMs to assess patient worsening and empower and educate patients on optimizing self-efficacy approaches.37 Platform selection should be based on accessibility, security and user-friendliness, especially in settings with variable internet connectivity. Digital literacy assessments for both patients and staff can inform targeted support, such as basic tutorials or visual aids. Platform selection should be based on accessibility, security and user-friendliness, especially in settings with variable internet connectivity. Digital literacy assessments for both patients and staff can inform targeted support, such as basic tutorials or visual aids. Artificial intelligence is establishing a foothold in supporting the diagnosis and management of diseases.103–105 This is particularly useful in RDs such as myositis, which are also characterized by significant clinical heterogeneity. Similarly, LLMs can be used for assessing outcome measures and may perform as good as human scorers, suggesting their potential use in recruiting patients for trials, scoring disease activity in the skin and muscle as well as triage of complex referrals.106–108 However, these need to be validated in larger studies. Integration of such technologies must be ethical, evidence-informed and sensitive to patient preferences. Clinicians should also be aware of regional telemedicine regulations, particularly when care extends across state or national boundaries.
Although there has been significant advancement in technology, the number of in-person educational meetings remains central as hybrid conference offerings continue to wane, placing cost, time and travel burdens on individuals. Embracing, improving and staying current on digital platform technical skills can provide cost and time efficiency, reach a wider target population and reduce environmental harm.109 Digital platforms can accelerate research collaborations and fortify mentorship and collegial relations in RDs. Digital technologies, though currently underused, possess awareness and skills training regarding the utility of special features.
Conclusion
Establishing a RD service is an important undertaking that can favourably impact health outcomes including quality of life for PLWRDs. Successful growth benefits from basic and resource-responsive planning as well as routinely re-evaluating the clinic’s scope of services, including diagnostics, treatment and support services. Multidisciplinary collaboration driven by patient-centred approaches is foundational. By remaining flexible to and harnessing available resources and opportunities, while learning from patient organizations and other community examples of RD clinics, clinicians inspired by a RD interest can establish and continue growing effective services and participate in the development of treatment options for PLWRDs.

